|
Polypeptides in the news

IX. a-Amino Acids, (Poly)peptides, and Proteins (continued)
One-letter system for long peptide sequences
The three-letter system for polypeptide representation described in
Part XVI is customarily used in texts and reports. However, a one-letter system has been developed for documents and files in which
brevity is important or computerization is intended. The listing
below shows the correlation of the two systems:
A (Ala), C (Cys), D (Asp), E (Glu), F (Phe), G (Gly), H (His), I
(Ile), K (Lys), L (Leu), M (Met), N (Asn), P (Pro), Q (Gln), R
(Arg), S (Ser), T (Thr), V (Val), W (Trp), and Y (Tyr).
The polypeptide illustrated in Part XVI:
Ala-Ser-Asp-Leu-Glu-Phe-Val
when converted to the one-letter system becomes:
A S D L E F V.
Protein and polypeptide conformation (folding)
Protein chains in their natural state spontaneously assume four
levels of conformational structure, the terminology of which can
present problems in translation. The four levels of conformation
are:
1. The primary structure - the sequence of amino acid residues that
make up the protein chain, as already discussed.
2. The secondary structure - the coiled or folded conformation assumed by a protein chain in its native environment.
3. The tertiary structure - the overall folded form assumed by a protein chain that has already folded or coiled into its secondary
structure.
4. The quaternary structure - the aggregation pattern of multiple protein chains.
Secondary structure of protein chains
The three principal forms of secondary structure of protein chains
are the a helix, the b sheet, and the b turn. Some (but by no means
all) of the terms that may be encountered in this discipline are
properly translated as follows:
a helix
b turn
b sheet
b-pleated sheet structure
amino acid sequence
antiparallel b-pleated sheet
coiled peptide chain
denatured protein
disordered region
fibrous protein
globular protein
microfibril
native structure
protein folding
protofibril
sheeted peptide chain
supracoiling
Fortunately it is not necessary to understand fully the meanings of
these terms to translate them properly! The terminology of proteins
is constantly evolving; new terms are invented every day.
All natural proteins are derived from a-amino acids. Currently being
explored are synthetic polypeptides made up of b-amino acid residues. Their interactions with living systems, their
conformations, their pharmacology, and their possible use in
medicine are being examined.
X. Other compounds of nitrogen
Nitriles (cyano compounds)
The loss of water from an amide leads to a nitrile, in which a
nitrogen atom is triple-bonded to carbon:
RC(=O)NH2 ® RCºN + H2O
The names of simple nitriles are derived from the root of the
trivial name of the related carboxylic acid. Alternatively, they can
be named as cyano-substituted hydrocarbons, as in the following
examples:
CH3CN = acetonitrile, ethanenitrile, or cyanomethane
C2H5CN = propionitrile, propanenitrile, or cyanoethane
n-C3H7CN = butyronitrile, butanenitrile, or 1-cyanopropane
(CH3)2CHCN = isobutyronitrile, 2-methylpropanenitrile, or 2-cyanopropane
CH2(CN)2 = malononitrile, dicyanomethane, or methanedicarbonitrile
CH2=CHCN = acrylonitrile
Numerous alternatives are available for many cyano-substituted
compounds. Some examples of commonly-used names are:
CH2=CHC(=O)OCH2CH2CN = cyanoethyl acrylate
CH2=C(CN)C(=O)OCH2CH3 = ethyl a-cyanoacrylate
HOCH2CH2CN = ethylene cyanohydrin or 3-hydroxypropionitrile
NCCH2CO2H = cyanoacetic acid
HN(CH2CH2CN)2 = bis(2-cyanoethyl)amine or dicyanoethylamine
Ureas and related compounds
The history of organic chemistry dates to 1828 when Friedrich Wöhler heated ammonium cyanate, an inorganic salt, and obtained urea,
previously believed to originate only from life processes:
NH4+OCN- ® H2NC(=O)NH2
Derivatives of urea abound. Urea when heated with diethyl malonate
yields barbituric acid:
| CH2(CO2C2H5)2 + CO(NH2)2 ® | 
|
Substituted malonic esters in this reaction lead to barbiturates, the use or abuse of which may have effects ranging from sedation to anesthesia to death.
Replacing the oxygen atom of urea with sulfur leads to thiourea H2NC(=S)NH2.
Urea-formaldehyde resins are manufactured in large tonnages for textile treatment, laminating compositions, fertilizers, and other
uses. Their production may proceed through dimethylolurea
[HOCH2NHC(=O)NHCH2OH] to give linear and/or branched and/or crosslinked polymers of the form HO[CH2NHC(=O)NH]nH, with branching
occuring at the nitrogen atoms.
The myriad types of organic compounds of nitrogen, when combined with their oxygen and sulfur derivatives, prohibit comprehensive
discussion. They will be treated individually below as they arise.
Their nomenclature is complex, individualistic at times, and even
well-versed chemists frequently have to refer to the literature for
help. When in doubt, an Internet search can often confirm or reject
a presumed translation in this area.
XI. Aromatic compounds
Aromatic hydrocarbons
In Part XIII with regard to cyclic compounds, we reserved
consideration of cyclohexatriene as a special case. The structure of
this compound, C6H6, can be written in two ways that differ only in the positions of the double bonds:
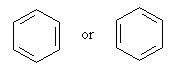
In such a case, as explained in detail in 1944 by George Wheland
(The Theory of Resonance and its Application to Organic Chemistry,
John Wiley and Sons, New York), neither of the two so-called Kekulé
structures shown above corresponds to the actual compound, benzene.
The molecule is in fact intermediate between the two Kekulé
structures, and all six carbon-to-carbon bonds are identical. This
"resonance" between different electron distributions as illustrated
classically occurs whenever a molecule can be depicted in two or
more ways differing only in the distribution of electrons, and with
little or no displacement of atoms. Resonance contributes greatly to
stability. For example, it is much more difficult to hydrogenate
benzene than cyclohexadiene. The electrons of benzene are termed "p-electrons" and are delocalized and distributed uniformly around the
ring. This resonance stabilization is called "aromaticity." The term
derives from the original discovery of the stability of these
compounds and their characteristic, generally pleasant, odors. The
aromatic nature of a ring system is often shown as a hexagon
containing a circle representing the delocalized electrons:
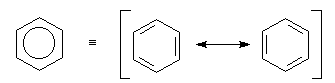
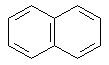
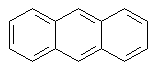
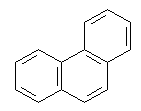
Benzene rings can be fused to one another or joined in series. The
fused systems are called binuclear, trinuclear, etc. They are also
resonance-stabilized. Examples of fused-ring systems are:
 |
 |
 |
| Naphthalene |
Anthracene |
Phenanthrene |
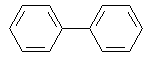
Examples of series-connected systems are:
 |
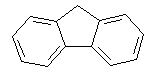 | |
Biphenyl | Fluorene | |
Part XVIII will continue the discussion of aromatic hydrocarbons.
|